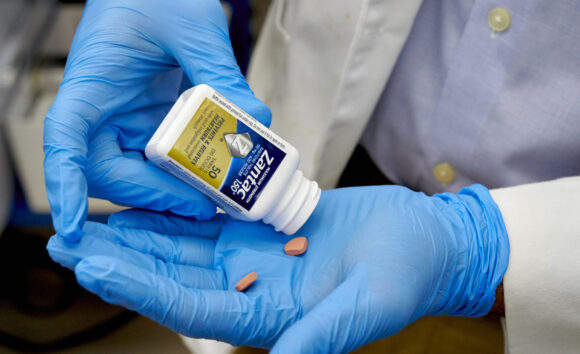
GSK Plc is facing a whistleblower lawsuit that could potentially cost it billions of dollars from the laboratory that revealed the presence of a probable carcinogen in Zantac, the heartburn medication that the UK drugmaker invented and turned into a blockbuster.
An amended complaint filed Monday contends that GSK hid Zantac’s cancer risks for decades while Medicare, Medicaid and other US government health programs covered prescriptions for the antacid. The lawsuit, which hasn’t been reported before, was brought by Connecticut-based Valisure, the independent lab that raised the alarm about Zantac’s cancer risks in 2019.
Big drugmakers already face some 80,000 state suits from users of the drug who say their cancers were caused by Zantac contaminated with NDMA, the dangerous chemical Valisure discovered. Bloomberg Intelligence estimates they could cost GSK as much $1.2 billion. The lab’s whistleblower suit addresses potential fraud on the part of the company and, if successful, would seek repayment of billions of dollars the government paid for Zantac, along with fines.
“Our argument is that every dollar the government — state or federal — paid for Zantac, was fraud,” Brent Wisner of Wisner Baum in Los Angeles, one of Valisure’s attorneys in the case, said in an interview. “It may rise to the level of posing an existential threat to GSK.”
GSK has said there’s “no consistent or reliable evidence” that Zantac increases the risk of cancer of any kind. Lyndsay Meyer, a US-based spokesperson for GSK, had no immediate comment on Valisure’s suit.
Whistleblower cases are typically brought by company employees or other insiders who report wrongdoing. Valisure filed the suit because it had non-public information about GSK when it discovered NDMA in Zantac. The lab also gave its findings to the US Food and Drug Administration several months before making them public.
Wisner and Jennifer Moore of Moore Law Group in Louisville, Kentucky, took on Valisure’s case this month. The pair are known for large verdicts they won against Bayer AG on behalf of people who claimed the company’s herbicide Roundup caused their cancers. The German company’s shares have lost about 70% of their value since investor concern arose about Bayer’s liability, eventually leading to the departure of former Chief Executive Officer Werner Baumann.
Confidential Settlements
GSK has reached a string of confidential settlements in Wisner’s and Moore’s state-court cases in California involving claims Zantac caused users’ cancers. The accords came on the eve of trial in some cases.
The whistleblower case, however, poses a different kind of financial risk than individual Zantac suits. Medicaid alone spent some $5.2 billion from 1991 to 2020 on Zantac, while Medicare’s costs over a seven-year period starting in 2012 were almost $800 million, according to the suit. Valisure’s lawyers will ask a judge to triple any recovery under provisions of states’ and the US government’s false-claim laws. GSK may also face fines of as much as $11,000 for each false claim it submitted, according to the filings.
Also called qui-tam complaints, whistleblower suits are filed under seal and given to the US Justice Department for review. Government prosecutors can intervene if they choose.
Valisure originally filed its whistleblower complaint in 2019, but the case was kept under seal until the government had a chance to review it. Laboratory officials filed the case on behalf of US taxpayers and those in 25 states.
A judge unsealed Valisure’s suit in March after the government declined to either join the suit or recommend dismissal. The amended complaint offers more details about the lab’s claims.
Potentially Lethal
In the suit, Valisure contends that NDMA is a potentially lethal carcinogen no matter how small the exposure and GSK officials knew Zantac could degrade into that substance in certain circumstances, but never warned consumers.
Just the second medication to garner more than $1 billion in annual sales, Zantac hit the US market as a prescription drug in 1983 before becoming an over-the-counter heartburn treatment in 1996. Some versions of the drug continued to require a prescription, such as those for infants.
French drugmaker Sanofi, which acquired over-the-counter Zantac in 2017, recalled the brand-name version shortly after Valisure released test results in 2019 showing elevated levels of NDMA in the medication and its generics. The lab’s research indicated the drug’s active ingredient, ranitidine, formed NDMA over time or at higher temperatures.
An odorless liquid chemical once used to make rocket fuel, NDMA is often found as a byproduct of manufacturing processes. The International Agency for Research on Cancer classifies it as a group 2A carcinogen, meaning that it’s probably carcinogenic in humans.
In 2020, the Food and Drug Administration confirmed Zantac and its generics could form NDMA and ordered drugmakers to take all versions of the medicine, including generics, off the market. Sanofi has since returned Zantac to store shelves but replaced ranitidine with famotidine, the active ingredient in competitor Pepcid, which hasn’t been shown to form NDMA.
Bloomberg reported last year that a study done in 1982 by GSK, then called Glaxo, showed the potential for Zantac to form NDMA. GSK kept the findings secret until sharing it with regulators after Valisure’s findings came out.
The case is Valisure v GlaxoSmithKline Plc, 19-cv-4239, US District Court, Eastern District of Pennsylvania (Philadelphia).
Photo: Photographer: Gabby Jones/Bloomberg
Topics Lawsuits
Was this article valuable?
Here are more articles you may enjoy.


 Florida Senate President Says No Major Insurance Changes This Year
Florida Senate President Says No Major Insurance Changes This Year  The $3 Trillion AI Data Center Build-Out Becomes All-Consuming for Debt Markets
The $3 Trillion AI Data Center Build-Out Becomes All-Consuming for Debt Markets  Lawyer for Prominent Texas Law Firm Among Victims ID’d in Maine Plane Crash
Lawyer for Prominent Texas Law Firm Among Victims ID’d in Maine Plane Crash  Uber Jury Awards $8.5 Million Damages in Sexual Assault Case
Uber Jury Awards $8.5 Million Damages in Sexual Assault Case 

